Blog
LAB EXPANSION
Valentia Analytical completed a move from Hayward to a beautiful location in Warm Springs, Fremont, CA. The new place is much sunnier. While we love the atmosphere of the new area, it is for the significantly larger size that moved. We are accommodating for new capabilities including CE-SDS, CIEF, ELISA and BIOASSAY. Most important, the…
Read MoreISO17025:2017 Annual Accreditation
Valentia Analytical successfully received its annual accreditation to ISO17025:2017 in August 2021. There were no observations and company received the highest positive outcome rating upon conclusion of the inspection. This success reflects its sustained Commitment to Quality!
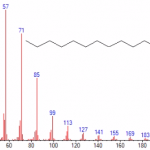
Read MoreGC-MS
The arsenal of analytical techniques was recently expanded upon the acquisition of a Gas Chromatograph coupled with a Mass Spectrometer (aka GC-MS). This provides our clients with the ability to characterize organic volatile compounds such as residual solvents or impurities. We are able to help identify unknown compounds by matching mass spectral data against a…
Read MoreISO/IEC17025 Accredited
Valentia Analytical is pleased to announce that it applied for and successfully passed the accreditation assessment to ISO/IEC17025:2017, General requirements for the competence of testing and calibrations laboratories. The assessment was performed by ANSI-ASQ National Accreditation Board (ANAB) which is an FDA recognized accreditation body. This was long process planned one year prior as documented…
Read MoreDocuSign and 21CFRPart11
Valentia Analytical has implemented DocuSign with an upgraded 21CFR11 module. DocuSign is a secure cloud-based application for the application of electronic signatures improved efficiency in obtaining approval of documents. This enables remote approvals which is particularly important in light of maintaining social distance standards related to COVID-19. The 21CFR11 module is a significant added cost.…
Read MoreCongratulations Justin Murio
We would like to congratulate Justin Murio, our intern who is beginning his academic endeavors at University of Oregon this fall. He intends to study Biology. He intends to contribute to this blog with learnings throughout his college career.
Read More